2/22/13
Today I started off my internship by cleaning out a sphalerite capsule from last week. I extracted the sulfur 34, but there as still a lot of sulfur stuck to the sphalerite mineral. I put the mineral in a beaker with some ethanol and placed it in the ultrasonic cleaner which sends ultrasound waves through the water to try and break up the sulfur on the mineral without damaging or scratching it in the process. While the sphalerite was being cleaned, Heather and I looked at the polished barite minerals that were still attached by crystal bond to a metal disk. After singling out a crystal with the smoothest surface, I carried the disk over to the hot plate. After the crystal bond had softened, I extracted the desired crystal and put it in a beaker with ethanol where it joined the sphalerite in the ultrasonic. While we waited for the ultrasonic to remove the crystal bond from the barite, Daniele, Heather and I went to the furnace. Barite is a softer mineral and not too many experiments have been done with sulfates so we decided to run our experiment at a low 300 degrees Celsius and to take the experiment out on Monday to ensure the sulfur wouldn't travel all the way through the barite before we took it out. After finding a furnace that was pretty close to 300 degrees we headed back to the lab. Daniele had to go to a conference call, so Heather and I looked at some of the other barite minerals under the microscope. We took some really cool pictures on Heather's phone and I hope to be able to put those up when she sends them to me. However, Heather did send me a picture last week of a capsule right after she took it out of the furnace.
As you can see, the sulfur is a reddish color when it comes out of the furnace! Usually it's a lot more red, but it has already started to cool in this picture. After looking at the barite minerals under the microscope, we filled a capsule with our desired barite mineral and some recycled sulfur 34 along with some new sulfur. We evacuated the capsule using the vacuum and while we were waiting, went to check on the furnace one last time. By the time we got back, our tube with the sulfur and barite was ready to be capsulized! Heather complemented me on my speedy efficiency as I quickly melted the tube to form a vacuum sealed capsule. We then placed the capsule in the furnace. With some time to kill, Heather took me to the room across from the lab where a lot of the pressurized furnaces are kept. We looked at some big metal tables that had been made in the workshop and were being sanded down. Apparently some of the soldering had some lead in them so we tried not to breathe too much. Heather showed me some salt that a mechanized mortar and pestle was grinding This salt will be pressurized into solid tubes which are used in the pressurized surfaces. An electrical current heats up the sample while the pressurized furnace simultaneously exerts a force upon it. The salt cells help to distribute this pressure fairly evenly throughout the experiment. The electrical current helps to make the salt soft, which helps to make sure the entire area surrounding the experiment is pressurized. Next week, we are going to look at some data from a former sphalerite experiment along with clean out the barite capsule. I'll try and put up some of the pictures I took today as soon as Heather sends them which will hopefully be before then.
Friday, February 22, 2013
Sunday, February 17, 2013
Brilliant Barite
2/15/13
Today I was a little late getting to my internship due to Emma idol. Even with a late start, Daniele, Heather and I were still able to get a bit done. We started cleaning out 2 previous capsules, one which contained galena and the other sphalerite. The sulfur within the galena capsule looked dark and not normal compared to previous capsules, so Daniele and I think some of the recycled sulfur-34 we have been using has been picking up contaminants. We decided to be a little more careful and selective with the recycled sulfur-34 to try and prevent contamination. The galena mineral was easy enough to extract from the capsule, but the sphalerite capsule was giving us a little trouble. Even though we had put the capsules on their sides when taking them out of the furnace to try and prevent the sulfur from encompassing the mineral in the capsule, the sulfur had still completely covered the sphalerite mineral. We began to slowly chip away at the unrelenting sulfur with a combination of a metal pick and ethanol. While we were soaking the capsule in ethanol, we decided to do an experiment with a new mineral. Sphalerite and galena are both sulfides, but we decided to use a sulfate. While a sulfide is just a mineral that contains sulfur, a sulfate contains both sulfur and oxygen molecules. Heather found some barite in a display case outside the lab and we used a makeshift chisel to extract some shards. After chiseling out some tiny chunks, we began to examine the shards under the microscope to see if we would have to polish them. While some of the barite contained little black flecks, which were particles the mineral had picked up throughout its growth, most of the shards would be experiment-ready with a little polishing. I headed over to the hot plate and melted some crystal bond on a tiny metal disk before setting the minerals. I polished them on a fine grain and when I was finished, Heather had just enough time to show me a video of a meteor that had landed in Russia. The compilation of videos was amazing and though the meteor did injure around 1000 people, the meteor in the video was pretty incredible to look at. You can watch the video here!
Hopefully next week we'll get a chance to make a capsule for the barite and put it in the furnace along with look at some more data.
Today I was a little late getting to my internship due to Emma idol. Even with a late start, Daniele, Heather and I were still able to get a bit done. We started cleaning out 2 previous capsules, one which contained galena and the other sphalerite. The sulfur within the galena capsule looked dark and not normal compared to previous capsules, so Daniele and I think some of the recycled sulfur-34 we have been using has been picking up contaminants. We decided to be a little more careful and selective with the recycled sulfur-34 to try and prevent contamination. The galena mineral was easy enough to extract from the capsule, but the sphalerite capsule was giving us a little trouble. Even though we had put the capsules on their sides when taking them out of the furnace to try and prevent the sulfur from encompassing the mineral in the capsule, the sulfur had still completely covered the sphalerite mineral. We began to slowly chip away at the unrelenting sulfur with a combination of a metal pick and ethanol. While we were soaking the capsule in ethanol, we decided to do an experiment with a new mineral. Sphalerite and galena are both sulfides, but we decided to use a sulfate. While a sulfide is just a mineral that contains sulfur, a sulfate contains both sulfur and oxygen molecules. Heather found some barite in a display case outside the lab and we used a makeshift chisel to extract some shards. After chiseling out some tiny chunks, we began to examine the shards under the microscope to see if we would have to polish them. While some of the barite contained little black flecks, which were particles the mineral had picked up throughout its growth, most of the shards would be experiment-ready with a little polishing. I headed over to the hot plate and melted some crystal bond on a tiny metal disk before setting the minerals. I polished them on a fine grain and when I was finished, Heather had just enough time to show me a video of a meteor that had landed in Russia. The compilation of videos was amazing and though the meteor did injure around 1000 people, the meteor in the video was pretty incredible to look at. You can watch the video here!
Hopefully next week we'll get a chance to make a capsule for the barite and put it in the furnace along with look at some more data.
Sunday, February 10, 2013
Individual Investigations
2/9/13
Heather and Daniele were both out of town today so Heather sent me an article giving a big-picture overview of the element sulfur along with an excel spreadsheet to look at some of the patterns formed with sulfur concentration as it diffused through pyrite. The article taught me some interesting new facts about sulfur, including its huge impact on all life forms. Sulfur is essential for life and in fact, a large portion of your body is made up of sulfur. Sulfur helps to synthesize proteins, which make up every part of your body including muscles organs and tissues. The article also gave me a bit of a history lesson, explaining how the US used to extract sulfur from the tops of salt domes. While the US has stopped extracting sulfur this way, it still remains the largest sulfur producer, recycling sulfur that is a by-product from petroleum refineries. This sulfur can be used in fertilizers, rubber, paper manufacturing and other industrial products. With sulfur also being utilized rather than simply an excess byproduct, companies have greatly reduced their sulfur emissions, choosing to process and utilize this resource instead. The article also talked about how there is some debate as to which sulfur isotopes appeared on Earth first. Theories predict that sulfur isotopes with greater mass were created later, but I want to ask Heather and Daniele more about this next week to better understand these theories.
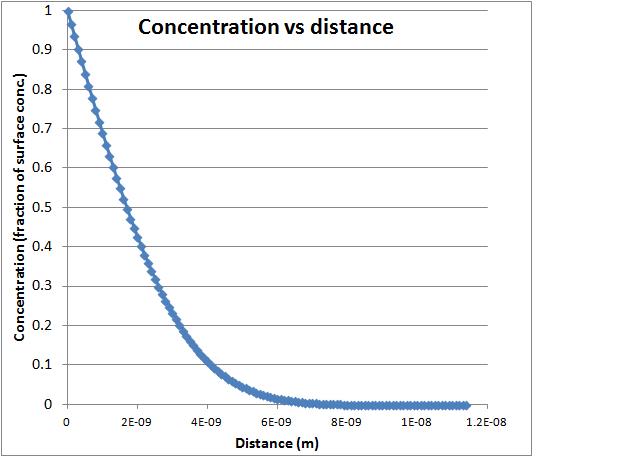
After reading the article I began to look over Heather's Excel sheet. The Excel sheet allowed me to manipulate a graph showing the concentration of sulfur through pyrite. It did this by showing the concentration of the sulfur through each layer of pyrite. As the sulfur got deeper within the pyrite, the concentration decreased, because it took more energy for the sulfur molecules to travel deeper within the pyrite. As the temperature decreased, the sulfur traveled through fewer layers, and this I assume, is because the molecules would have less energy. As I increased the time the sulfur was heated with the pyrite, the slope of the graph got steeper, showing that the sulfur was able to penetrate through more layers over time. If the temperature was increased and the time decreased, the graph never leveled out. The graph below eventually flatlines, when the sulfur concentration reaches zero because it can travel no further through the pyrite. If the temperature was increased, the sulfur molecules would have more energy to travel through the pyrite, but because the time was decreased, they would not have enough time to travel to the maximum depth. The third value I could manipulate on the graph was called D at temperature, which I'm assuming is the average distance traveled through the pyrite at a certain temperature, but I'll have to ask Heather and Daniele next week to make sure. Here is a picture of the graph I was manipulating. You can see how the graph levels off where the concentration approaches zero as it goes deeper within the pyrite.
Heather and Daniele were both out of town today so Heather sent me an article giving a big-picture overview of the element sulfur along with an excel spreadsheet to look at some of the patterns formed with sulfur concentration as it diffused through pyrite. The article taught me some interesting new facts about sulfur, including its huge impact on all life forms. Sulfur is essential for life and in fact, a large portion of your body is made up of sulfur. Sulfur helps to synthesize proteins, which make up every part of your body including muscles organs and tissues. The article also gave me a bit of a history lesson, explaining how the US used to extract sulfur from the tops of salt domes. While the US has stopped extracting sulfur this way, it still remains the largest sulfur producer, recycling sulfur that is a by-product from petroleum refineries. This sulfur can be used in fertilizers, rubber, paper manufacturing and other industrial products. With sulfur also being utilized rather than simply an excess byproduct, companies have greatly reduced their sulfur emissions, choosing to process and utilize this resource instead. The article also talked about how there is some debate as to which sulfur isotopes appeared on Earth first. Theories predict that sulfur isotopes with greater mass were created later, but I want to ask Heather and Daniele more about this next week to better understand these theories.
After reading the article I began to look over Heather's Excel sheet. The Excel sheet allowed me to manipulate a graph showing the concentration of sulfur through pyrite. It did this by showing the concentration of the sulfur through each layer of pyrite. As the sulfur got deeper within the pyrite, the concentration decreased, because it took more energy for the sulfur molecules to travel deeper within the pyrite. As the temperature decreased, the sulfur traveled through fewer layers, and this I assume, is because the molecules would have less energy. As I increased the time the sulfur was heated with the pyrite, the slope of the graph got steeper, showing that the sulfur was able to penetrate through more layers over time. If the temperature was increased and the time decreased, the graph never leveled out. The graph below eventually flatlines, when the sulfur concentration reaches zero because it can travel no further through the pyrite. If the temperature was increased, the sulfur molecules would have more energy to travel through the pyrite, but because the time was decreased, they would not have enough time to travel to the maximum depth. The third value I could manipulate on the graph was called D at temperature, which I'm assuming is the average distance traveled through the pyrite at a certain temperature, but I'll have to ask Heather and Daniele next week to make sure. Here is a picture of the graph I was manipulating. You can see how the graph levels off where the concentration approaches zero as it goes deeper within the pyrite.
Saturday, February 2, 2013
Perplexing Programs
2/1/13
Daniele and I hopped right into things, cleaning out a capsule from last week that Daniele had removed from the furnace. Daniele had let the molten sulfur cool with the capsule on its side, so this time, we avoided the problem of having the mineral buried within a solidified chunk of sulfur. After scraping out the tube with ethanol and a spatula and placing the powdery liquid under a heat lamp, I had successfully recycled our sample of sulfur-34. We then started to make anothetor capsule with one of the galena crystals Heather had polished. Using the tubes that I had half sealed last week, we hooked up the capsule with the galena and sulfur to the vacuum and then formed our capsule by melting the other end up the tube. Luckily, I have not been setting things on fire lately and Daniele says I am getting better. Next we went to the furnace to put in our new sample and adjust a different furnace for a sphalerite experiment we were going to run. After inserting the capsule and changing the temperature of a different furnace we headed back to the lab to begin making our sphalerite sample. I used the last of the recycled sulfur-34 and some of the new sulfur Daniele had gotten to have enough sulfur to promote maximum diffusion through the sphalerite crystal. We hooked the sphalerite up to the vacuum and then headed off down the hall where I began to look at the simulation websites.
Though the simulation was confusing at first, I eventually began to get the gist of things.
The above is one of the graphs we were looking at. The blue line is the simulation generated by the computer and the darker line are the points found by the accelerator when examining a mineral. By changing certain things like the ratio of the sulfur to the other element that makes up the sulfide mineral or the thickness of the layer, we can make the simulation more closely match the actual graph. After editing layer after layer of the mineral to modify the simulation, the simulation will finally match the data and we can determine the concentration of sulfur molecules at each depth of the mineral. After determining the concentration of the sulfur at each layer, we can then make a graph showing this concentration slowly decreasing as you travel deeper within the molecule. With this data, we can begin to calculate the rate of the diffusion of the sulfur which will provide us with the necessary data for our experiment on sulfur diffusion. The graph above shows the data from a sphalerite mineral. The left side of the graph shows the smaller sulfur molecules while the right shows the larger zinc molecules that also make up the sphalerite sample.The little bump in the graph between the two represents the sulfur-34, the sulfur isotope we are working with in our experiment. Though I still only have a basic understanding of how to modify the simulation graph, after about an hour of practice with Heather and Daniele, I started getting better at manipulation numbers and adding data. Next week, I will not meet with Heather and Daniele, but Heather is going to send my articles and some calculations I can work on.
Daniele and I hopped right into things, cleaning out a capsule from last week that Daniele had removed from the furnace. Daniele had let the molten sulfur cool with the capsule on its side, so this time, we avoided the problem of having the mineral buried within a solidified chunk of sulfur. After scraping out the tube with ethanol and a spatula and placing the powdery liquid under a heat lamp, I had successfully recycled our sample of sulfur-34. We then started to make anothetor capsule with one of the galena crystals Heather had polished. Using the tubes that I had half sealed last week, we hooked up the capsule with the galena and sulfur to the vacuum and then formed our capsule by melting the other end up the tube. Luckily, I have not been setting things on fire lately and Daniele says I am getting better. Next we went to the furnace to put in our new sample and adjust a different furnace for a sphalerite experiment we were going to run. After inserting the capsule and changing the temperature of a different furnace we headed back to the lab to begin making our sphalerite sample. I used the last of the recycled sulfur-34 and some of the new sulfur Daniele had gotten to have enough sulfur to promote maximum diffusion through the sphalerite crystal. We hooked the sphalerite up to the vacuum and then headed off down the hall where I began to look at the simulation websites.
Though the simulation was confusing at first, I eventually began to get the gist of things.
The above is one of the graphs we were looking at. The blue line is the simulation generated by the computer and the darker line are the points found by the accelerator when examining a mineral. By changing certain things like the ratio of the sulfur to the other element that makes up the sulfide mineral or the thickness of the layer, we can make the simulation more closely match the actual graph. After editing layer after layer of the mineral to modify the simulation, the simulation will finally match the data and we can determine the concentration of sulfur molecules at each depth of the mineral. After determining the concentration of the sulfur at each layer, we can then make a graph showing this concentration slowly decreasing as you travel deeper within the molecule. With this data, we can begin to calculate the rate of the diffusion of the sulfur which will provide us with the necessary data for our experiment on sulfur diffusion. The graph above shows the data from a sphalerite mineral. The left side of the graph shows the smaller sulfur molecules while the right shows the larger zinc molecules that also make up the sphalerite sample.The little bump in the graph between the two represents the sulfur-34, the sulfur isotope we are working with in our experiment. Though I still only have a basic understanding of how to modify the simulation graph, after about an hour of practice with Heather and Daniele, I started getting better at manipulation numbers and adding data. Next week, I will not meet with Heather and Daniele, but Heather is going to send my articles and some calculations I can work on.
Subscribe to:
Comments (Atom)